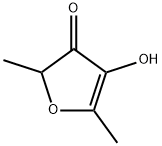
Furaneol:Activity, Application and Chemical Studies
Feb 28,2023
General Description
As the king of spices, furaneol exists in food, tobacco and beverages, and has an obvious aroma-modifying effect when the flavor threshold is 0.04ppb, so it is widely used as a flavoring agent for food, tobacco and beverages;[1]As an import aroma chemicals and are considered key flavor compounds in many fruit. Due to its attractive sensory properties they are highly appreciated by the food industry. Furaneol is formed by addition of a C1 fragment, derived from glycine by Strecker degradation, to a diketose derived from a C5 carbohydrate. The furanones have been identified in a multitude of processed food. The levels of furanones produced depend on the type and amount of sugars and amino acids available in the raw food material, the pH value and the heating regime. Besides their chemical formation during thermal treatment of food 4-hydroxy-3(2H)-furanones are biosynthesized by plants, microorganism, and insects. Its caramel-like flavor is associated with a planar enol-oxo group of a cyclic dicarbonyl derivative able to form strong hydrogen bonds.Researchers found that it was unstable at all pH values by observing the the stability of furaneol. Furaneol have been isolated as natural products from many different fresh fruits such as strawberry, raspberry, tomato, kiwi, lychee, and snake fruit.[2]

Figure 1 furaneol powder
Preparation
The method for synthesizing furaneol, comprising the following two steps:
Step 1. Preparation of conjugate:
1). Add 20 g of glyoxal and 20 ml of acetic acid to the three-necked flask from the funnel in turn, then turn on the automatic stirring device, and maintain the temperature of the water bath in the range of 25 to 40°C for 15 minutes. Then add 4g of zinc powder to the three-necked flask through the funnel, then add 4g of zinc powder to the three-necked flask every 15 minutes, and add 16g of zinc powder to the three-necked flask in total. Add ice cubes to the water bath to maintain the temperature of the water bath in the range of 25-40 °C.
2). After the addition of zinc powder, continue to keep the temperature of the water bath in the range of 25 to 40°C, and stir the reaction for 1.5 hours; the excess zinc powder removed by filtration is stirred with 1 to 2% of dilute hydrochloric acid for 1 to 3 minutes , filter, rinse the filter cake with water, ethanol and ether in turn to obtain dry and activated zinc powder, and recovering the zinc powder in the reaction process is conducive to saving resources and reducing the production cost of furanone;
3).after the completion of the reaction, the excess zinc powder and the saturated part of the zinc acetate precipitated are removed by filtration under reduced pressure, the filtrate is under the condition of lower than 55°C on the rotary evaporator, the aqueous solution is evaporated to dryness under reduced pressure, and leached twice with 80 mL of ethyl acetate , filtered to remove the zinc acetate crystals, the filtrate was dried, and the solvent ethyl acetate was recovered by atmospheric distillation to obtain 19 g of light yellow oily viscous liquid; 80mL of ethyl acetate was leached in two steps, the amount of ethyl acetate for the first time was 50ml, and the amount of ethyl acetate for the first time was 50 ml. The amount of secondary ethyl acetate is 30ml, and the leaching temperature is controlled within the range of 35 to 40°C;
4). Add a mixture of 20mL of n-butanol and 16 mL of petroleum ether to the light yellow oily viscous liquid, crystallize at a low temperature of -5 to 0 °C, filter and dry to obtain 9.4 g of threo-3,4-dihydroxy-2,5- Hexanedione, the melting point of threo 3,4-dihydroxy-2,5-hexanedione is 90°C, and the melting point of the prepared erythro-3,4-dihydroxy-2,5-hexanedione is 60°C, A vacuum drying oven is used for drying.
5).depressurize the filtrate after separating threo 3,4-dihydroxy 2,5-hexanedione, then remove the solvent to obtain a yellow oily liquid, then add a mixture of chloroform and petroleum ether, add erythro 3, 4-Dihydroxy-2,5-hexanedione was used as a seed crystal and crystallized at low temperature to obtain erythro-3,4-dihydroxy-2,5-hexanedione by filtration; the prepared conjugates included threo-3,4-dione Hydroxy-2,5-hexanedione and erythro-3,4-dihydroxy-2,5-hexanedione, of which threo-3,4-dihydroxy-2,5-hexanedione accounted for 83% of the total , erythro-3,4-dihydroxy-2,5-hexanedione accounted for 18% of the total;
Step 2. Prepare furanone: add 10ml of Na2HPO4 aqueous solution to the prepared conjugate, react at a certain temperature for 24 hours, add acetic acid to the solution to adjust the pH value of the solution, and then let the reacted solution stand at room temperature After cooling and standing for a period of time, the oil phase and the water phase were separated, and the water phase was concentrated and recovered sodium dihydrogen phosphate and returned to the cyclization reaction, and the waste water was discharged; Product, the crude furanone is obtained through freezing crystallization and centrifugal separation to obtain the crude furanone, and the crude furanone is dissolved in ethanol and ethyl acetate for extraction. The volume ratio of ethanol and ethyl acetate is 1:1.1-8.2, and filtered out. After the impurities are removed, freeze crystallization and centrifugal separation to obtain the finished furanone, and dry the wet furanone to obtain the finished furanone.[1]
Activity
The anti-oxidative function of furaneol. The protective activity of furaneol against superoxide radicals in lens tissue contributed to inhibiting the onset of spontaneous cataracts.Levels of thiobarbituric acid-reactive substances in lung were remarkably increased, and those in kidney and liver were slightly decreased by supplementation of furaneol.The study of the de-pigmenting capacity for furaneol showed that furaneol suppressed the phosphorylation of cAMP response element binding protein, which is induced by protein kinase A and suggested that furaneol may be an effective inhibitor of hyperpigmentation.
Furaneol also exhibited broad spectrum antimicrobial activity in an energy-dependent manner without hemolytic effect on human erythrocytes. And furaneol may have potential as an anti-infective agent in human microbial infections.Radiotracer studies identified carbohydrates as the natural precursors of furaneol and its derivatives.Furanones are also produced by yeast, bacteria and plants and show different physiological functions which are probably due to their redox activity.[2]
Application
Furaneol was defined as one of the key odorants in pineapples and has been frequently found in wines. Furaneol has been identified as one of the key odorants in Spanish aged red wines and in Bordeaux red wines. Furaneol also contributes to the aroma of human breast milk and to milk products such as nonfat dry milk and sweet whey powder.[2]
Reference
[1]Chen Q.Preparation of furaneol.[P]CN113773286. 2021,12,10.
[2]Schwab W. Natural 4-hydroxy-2, 5-dimethyl-3 (2 H)-furanone (furaneol?)[J]. Molecules, 2013, 18(6): 6936-6951.
- Related articles
- Related Qustion
- Furaneol: Biosynthesis and Pharmacological Activities May 10, 2024
Furaneol, synthesized by yeast and bacteria in fermented soy sauce, possesses antimicrobial properties and activates odor receptors.
- Unlocking the Flavor Potential of Furaneol: Applications and Toxicity Considerations in the Food Industry Dec 22, 2023
Furaneol, a widely used flavoring agent, has a sweet aroma but raises toxicity concerns due to adverse effects in animal studies, mutagenicity, and genetic damage.
Di-Fluoro ethylene carbonate can improve the cycle performance, high temperature performance and flame retardant performance of the electrolyte, is a lithium battery electrolyte additive.....
Feb 28,2023APIGlucose oxidase is an enzyme found in molds such as Penicillium notatum and honey. It can catalyze D-glucose and O2 to produce D-gluconic acid (δ- Lactone) and H2O2.....
Feb 28,2023APIFuraneol
3658-77-3You may like
- Strawberry Flavor Furanone
-

- $120.00 / 1kg
- 2024-05-16
- CAS:3658-77-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Furaneol
-

- $18.00 / 10kg
- 2024-05-07
- CAS:3658-77-3
- Min. Order: 1kg
- Purity: 99.9
- Supply Ability: 5000
- Furaneol
-

- $0.00 / 1kg
- 2024-05-07
- CAS:3658-77-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000kg